Academix
A Non-profit Organization to Help Students Study, Explore, and Engage
Oxyanions
Br I Cl Mn N C
These elements form oxyanions with the O3 baseline (n=3), have the following charges regardless of the number of oxygens present.
| Element | Br | I | Cl | Mn | N | C |
| Charge | 1- | 1- | 1- | 1- | 1- | 2- |
| Resulting base (n = 3) oxyanion | BrO3- | IO3- | ClO3- | MnO3- | NO3- | CO32- |
Cr As P S
These elements form oxyanions with the O4 baseline (n=4), have the following charges regardless of the number of oxygens present.
| Element | Cr | As | P | S |
memorize: this triangle helps remembering the charges. |
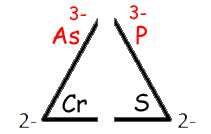 |
| Charge | 2- | 3- | 3- | 2- | ||
| Resulting base (n = 4) oxyanion | CrO42- | AsO43- | PO43- | SO42- |
Discussions on Charges Within the Same Group
| Example, the charge of SO42- is -2. | ||
| The charge of SO32- is also -2. |
Academix: Study, Explore, Engage...
Copyright © 2025 Academix. All Rights Reserved.