Academix
A Non-profit Organization to Help Students Study, Explore, and Engage
You have chosen compound with one or more Hydrogens. Often this will be an acid but not always.
We use the word "acid" to expresses the presence of H in the formula, but only if the compound is actually an acid. The first naming scheme below always works, the second works for oxyacids only.
Naming Inorganic Compounds: Try a new formula
Click here to see the Legend. See also: typical oxidation numbers
 |
or |
  an Oxyanion |
|||
 |
 |
||||
|
Naming Scheme 1: A polyatomic combination (other than oxyanions). |
or | Naming Scheme 2: The polyatomic ion is an oxyanion. The resulting formula is an Oxyacid (= Oxoacid), and thus makes no mention of the Hydrogen in the name. This is unlike Binary Acids which do use the word Hydro- to designate the Hydrogen. |
|||
 |
morphed oxyanion name + acid | ||||
| This naming scheme always works, and can be applied equally to oxyanions. |
 |
||||
 |
compose the morphed oxyanion name: | ||||
| If there is only one H present, then: | There are n Hydrogen atoms, and n > 1, then: | if the original oxyanion has either suffix: | |||
|
|
or |
|
 |
 |
|
 |
 |
||||
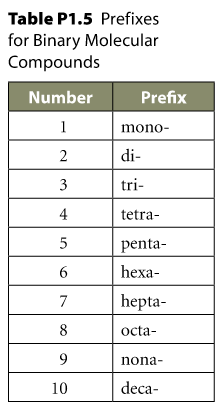
| Greek Prefix + Hydrogen +
|
-ATE suffix | -ITE suffix | |||
| remove this suffix | remove this suffix | ||||
| Hydrogen +
|
|||||
| Never prefix the first hydrogen if there is only one such, with mono-. | and replace w/ -IC suffix | and replace w/ -OUS suffix | |||
| next append the word acid. | |||||
Examples
| Oxyanion in the formula | Scheme 2 not always applicable. | Scheme 1 can always be applied. | ||||
| HCO3- |
CO32- = Carbonate | This is
an ion, derived from an acid (deprotonated). It is mildly alkaline
(basic) and thus not an acid. |
Hydrogen Carbonate | |||
| H2CO3 |
CO32- = Carbonate | the acid name = Carbonic acid | Dihydrogen Carbonate | |||
| H2SO4 | SO42- = Sulfate | the acid name = Sulfuric acid | Dihydrogen Sulfate | |||
| H3PO4 | PO43- = Phosphate | the acid name = Phosphoric acid | Trihydrogen Phosphate | |||
| HCN | weakly acidic: Hydrocyanic acid |
Hydrogen Cyanide |
Notes
Use Scheme 1 if you are not sure if the compound is an acid.
Academix: Study, Explore, Engage...
Copyright © 2025 Academix. All Rights Reserved.