A Non-profit Organization to Help Students Study, Explore, and Engage
Multiple atoms on the left side form a polyatomic ion.
Click the item that fits on the right side of the formula.
Naming Inorganic Compounds: Try a new formula
Click here to see the Legend. See also: typical oxidation numbers
Facts about Polyatomic Combinations:
Most polyatomic ions act like non-metals (negative charges) and form ionic compounds with metals. In that case change the positioning within the formula as follows:
When the polyatomic ion is positively charged (for ex. NH4+) then it appears on the left side of the formula as follows:
Polyatomic combinations with brackets and multiple instances such as these
![]()
![]() and
and ![]()
![]()
do not contribute Greek Numerals to the polyatomic part of the name, regardless if they act like a metal or non-metal.
Examples:
| (NH4)2S | Ammonium Sulfide | |
||
| Sr(H2PO4)2 | Strontium Dihydrogen Phosphate | |
||
| Ca2(NO3)2 | Calcium Nitrate | |
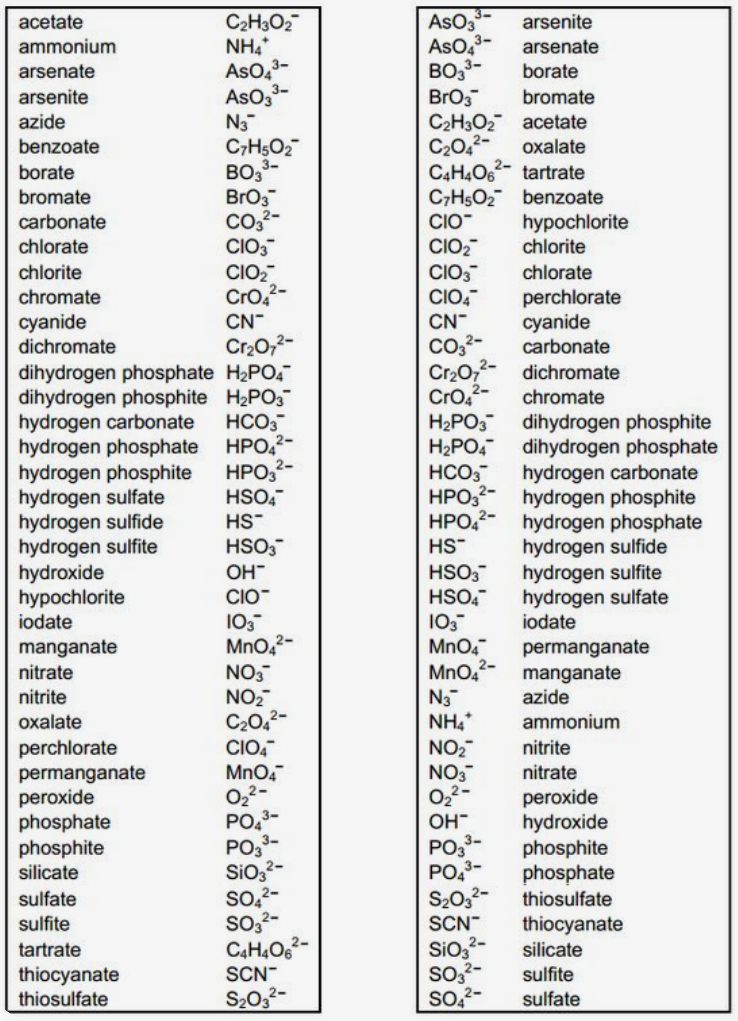
On its own,![]() can
be any of the following combinations:
can
be any of the following combinations:
polyatomic examples #3 (Table P1.6)
or oxyanions![]()
![]()
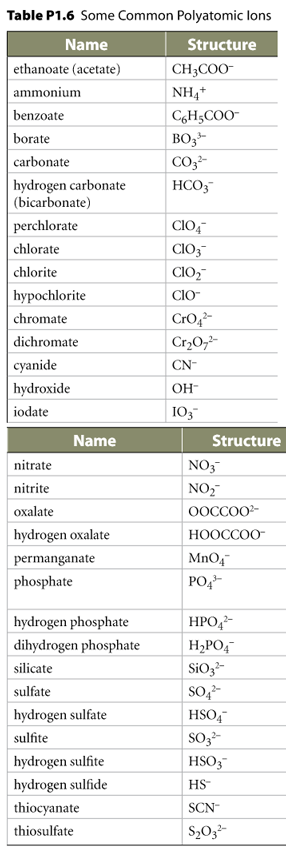
See your Alberta Chemistry 20 Textbook pdf.
Read the "Names and Formulas for Binary Compounds" section on page 10 or see the theory here.
Academix: Study, Explore, Engage...
Copyright © 2025 Academix. All Rights Reserved.

