Academix
A Non-profit Organization to Help Students Study, Explore, and Engage
You have selected a Hydroxide.
Hydroxides can be either acids or bases.
Hydroxides can be either acids or bases.
Naming Inorganic Compounds: Try a new formula
Click here to see the Legend. See also: typical oxidation numbers
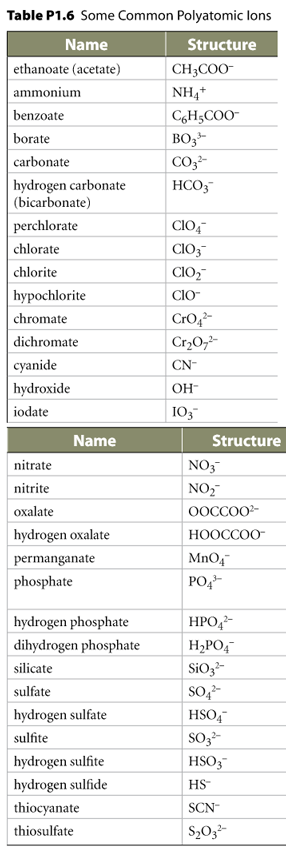
 stem stem This part of the name is composed based on several possibilies. Here are a few:  = =   = =   = =   = =   = an ion
listed in Table P1.6 on the right = an ion
listed in Table P1.6 on the right |
Hydroxide | The number of instances in a group has no impact on the name. I.e. (OH)2 is named the same as OH. | ||
Examples
Examples:
| Cu2CO3(OH)2 | Copper (II) Carbonate Hydroxide. This is a salt, also known as Malachite. |
 = Cu2CO3 = Cu2CO3 = OH = OH |
|
| NH4OH | Ammonium Hydroxide. This is a base. |
 = NH4 = NH4 = OH = OH |
Academix: Study, Explore, Engage...
Copyright © 2025 Academix. All Rights Reserved.