Academix
A Non-profit Organization to Help Students Study, Explore, and Engage
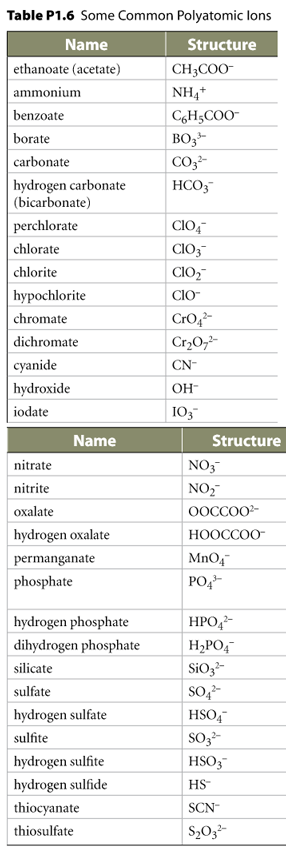
Multiple atoms that form a polyatomic ion in combination with a metal require the metal to be in the first (left) position.
Naming Inorganic Compounds: Try a new formula
Click here to see the Legend. See also: typical oxidation numbers
Naming: No Greek numerals are used in this case.
Next, examine options for
 . Each
part contributes to the name according to its position.
. Each
part contributes to the name according to its position.
 |
 |
||||
|
|
|
|
|||
 is a
transition metal is a
transition metal |
 is a
non-transition metal is a
non-transition metal |
place a space between
 and and
 . . |
The name of the polyatomic ion without the word "ion". | ||
| M stem (I) + π stem | M stem + π stem | ||||
|
|
|||||
|
Example 1: Au(ClO4)3 Gold(III) Perchlorate
|
Example 1: NaOH = Sodium Hydroxide |
 = Na = Na =
OH =
OH |
|||
|
Example 2: Fe(NO3)3 Iron(III) Nitrate
|
Example 2: Na2SO4 = Sodium Sulfate |
 = Na = Na =
SO4, an oxyanion =
SO4, an oxyanion |
|||
|
Example 3: Sr(H2PO4)2 Sr = an alkaline earth metal, group II. H2PO4 = dihydrogen phosphate, so the resulting name does not require the "ide" suffix. Notice the 2 (red) subscript in (H2PO4)2 does not have any impact on the name. The final name is: Strontium Dihydrogen Phosphate. |
 = Sr = Sr = H2PO4 = H2PO4 |
||||
|
Example 4: KCN = Potassium Cyanide |
 = K = K = CN- = CN- |
||||
|
Example 5: Mn(ClO2)2 = Manganese Chlorite |
 = Mn = Mn = ClO2, an oxyanion = ClO2, an oxyanion |
||||
|
Example 6: Na2CO3 = Sodium Carbonate |
 = Na = Na = CO3, an oxyanion = CO3, an oxyanion |
||||
|
Example 7: Ca2(NO3)2 = Calcium Nitrate |
 = Ca = Ca =
NO3, an oxyanion =
NO3, an oxyanion |
See below common examples of
![]() that
are not oxyanions.
that
are not oxyanions.
Notice all the ions below are negative except ammonium.
| C 2H3O2- | acetate | if you remember
C2H4O2 = acetic acid (= ethanoic acid = vinegar), the acetate ion is one H short of that. |
| O22- | peroxide | this is not the same as the O2 molecule which is not charged. |
Academix: Study, Explore, Engage...
Copyright © 2025 Academix. All Rights Reserved.

